What to Know about the pH of Drinking Water

The pH of water measures the degree of its acidity or basicity (alkalinity), and is expressed on a scale of 0 to 14. Water with a pH of 7 is neutral. pH less than 7 is acidic, with 0 the most acidic. More than 7 is basic or alkaline, with 14 the most basic. The EU Drinking Water Directive considers pH an “indicator parameter” which should be monitored, not a quality parameter that must be met. It recommends a range of 6.5 to 9.5 for public water systems, 4.5 to 9.5 for bottled water, and states that carbonated waters may be even lower.
According to the United States Environmental Protection Agency, the World Health Organization’s (WHO) Guidelines for Drinking Water Quality (CDWQ), and the EU’s Drinking Water Directive (DWD), the pH of water usually has no direct impact on the health and safety of consumers. Yet it is one of the most important parameters of water quality that has to be monitored because pH affects the way water interacts with its environment.
Such changed interactions might have a negative impact on the appearance, taste, and odor of drinking water and might indicate problems in water production and distribution systems that are dangerous to consumer health. However, not only within but even well beyond the EU’s recommended indicator parameter pH ranges of 4.5 or lower to 9.5, scientific research does not support claims that water acidity or alkalinity are harmful or beneficial to health in and of itself.
Table of Contents
What Is pH?
pH is a measure of acidity or alkalinity, with 0 the most acidic, 7 neutral, and 14 the most alkaline. The technical definition according to Lehninger’s Principles of Biochemistry is that pH is equal to the negative logarithm of hydrogen ion concentration in a solution. In non-mathematical terms, pH measures the relative equilibrium between positively and negatively charged ions in a solution. In water, these charged molecules are produced when chemical compounds dissolve in it.
The term pH stands for either potential hydrogen or power of hydrogen, with the H capitalized because it represents the symbol of the element. Whether power or potential was the intention of the scale’s creator Søren Sørensen is uncertain. Sørensen was the director of Danish beer producer Carlsberg’s chemistry laboratory and was searching for a way to measure acidity in order to ensure consistent beer quality when he came up with the measure in 1909.
The primary ions used to calculate the pH of water are Hydrogen (H+) and Hydroxyl (OH-). Water that contains more hydrogen (H+) ions has a low pH and is acidic. Water that contains more hydroxyl (OH-) ions has a high pH and is basic or alkaline. Water with equal hydrogen and hydroxyl ions is neutral with a pH of 7.
The pH scale is logarithmic, which means a change of 1 unit represents a 10 fold change in pH. So water with a pH of 6 is 10 times more acidic than water with a pH of 7, and water with a pH of 5 is 100 times more acidic than water with a pH of 7. Likewise, water with a Ph of 8 is ten times more alkaline than water with a pH of 7.
Because the number of hydrogen or hydroxyl ions is not limited to 10^7, pH below 0 or above 14 is possible. An October 2016 article in the Journal of Chemical Education points out there is commercially available hydrogen chloride (HCl) with a pH of -1.1, sodium hydroxide (NaOH) with a pH of 15, and even hot springs around Russia’s Ebeko Volo with a pH of -1.7.
This video from educational consultant and science teacher Paul Andersen explains the chemistry of pH in greater detail.
What Is the Importance of the pH of Drinking Water?
The importance of the pH of drinking water is that it alters the solubility and behavior of minerals and heavy metals that water comes in contact with. In some cases, this results in corrosion of pipes and equipment in plants and distribution systems the water passes through. A drastic change in pH turns normally innocuous chemical constituents of water into toxic substances. This negatively impacts the physical characteristics of water such as color, odor, turbidity, and taste as well as being potentially toxic to the consumer’s health.
The pH of water alone is relatively unimportant when it comes to the safety of drinking water itself, even more so for bottled water than municipal tap water. The ions which impart acidity and alkalinity to water are weak acids and bases, and as such, the direct effects of drinking water pH are negligible at best. The importance of pH should not be viewed alone but as part of a larger picture which includes the concentrations of minerals and metals, electrical conductivity, oxygen concentration, and temperature. So long as water at the point of consumption tests at safe levels for unhealthy contaminants, pH is not generally a matter of concern.
How Does pH Affect Drinking Water?
pH affects drinking water by changing its aesthetic characteristics, taste in a very slight manner, corrosiveness, and efficiency of disinfection processes. pH as a parameter on its own has only minor influences on the safety or taste of drinking water itself, as humans are tolerant to a broad range of water pH values. This is shown by the fact that no governmental or international organization requires bottled water producers or municipal systems to comply with predetermined pH levels. The United States Environmental Protection Agency (EPA) lists pH among its secondary water standards, which only have either cosmetic or aesthetic issues but do not pose any health risks. The EU’s Drinking Water Directive similarly lists pH only as an “indicator parameter” for observation and not necessary for health control purposes.
The four areas that pH has the most effect on in drinking water are the following.
- Aesthetic characteristics: At extreme levels, pH affects the physical characteristics of water such as color, odor, turbidity, or taste. This is rare and often involves interaction with other factors such as corrosiveness, but it occurs.
- Taste: Low pH water takes on a sour or metallic taste, and high pH a bitter or baking soda taste. That bitter taste is passed on to coffee or tea as well. Michael Mascha of the Fine Water Society states that a significant acidic taste does not begin until water reaches a pH of 5 or below. Between a pH of 5 and 10, he assesses only 5% of the flavor profile is driven by pH with the rest the result of mineral content and carbonation. Out of 167 still bottled waters which the Fine Water Society has data on, none have a pH below 5, and amongst sparkling waters only Voss sparkling water is below the level at 4.8.
- Corrosiveness: pH is one factor that causes water to be more or less corrosive. Higher corrosiveness causes metal ions to leach into water. But pH is only one factor and water of all pH levels is corrosive in varying degrees. Electrical conductivity, oxygen concentration, temperature, minerals, and other factors all play a role. A neutral pH 7 distilled water is corrosive because it lacks minerals and more readily absorbs materials it contacts including metals. The WHO says water under pH 6.5 is corrosive, and Dr. Robert Ashley of the UCLA Health system states that pH 8.5 water causes calcium and magnesium buildup in pipes which is corrosive. The topic is complex, and the leaching of excessive metal ions into water causes serious gastrointestinal, renal, hepatic, and central nervous system problems. But this is exceedingly rare and test results for metals and contaminants are the proper indicators to check for, not pH in itself.
- Efficiency of disinfection: Chlorine is the most common water disinfection agent in the world and pH impacts its effectiveness. The WHO’s Guidelines for Drinking Water Quality state that pH should be under 8 for chlorine to be effective for the disinfection of water supplies. At a pH of 6.5, 92% of the disinfection agents from chlorine are present, but at a pH of 8.5, this drops to 11%.
It should be noted that claims of high pH alkaline water having beneficial health effects from offsetting supposed negative acidity levels in the body do not have scientific support. Registered dietician Beth Czerwony RD of the Cleveland Clinic states points out that acids in the stomach immediately neutralize the impact of alkaline water, lungs remove excess carbon dioxide if blood has become too acidic, and that in general “Your body is perfectly capable of doing what it needs to adjust your pH levels”.
The fact that other foods and beverages we ingest have pH levels far beyond any water we drink is a good sign of the safety of water at nearly every level it is supplied. Here is a drinking water pH level chart alongside some other product pH levels.

How Can You Test the pH Level of Drinking Water?
According to the U.S. Geological Survey's (USGS) Water Science School, the pH level of drinking water is tested using either optical or potentiometric methods.
Potentiometric methods are the most commonly used ways to test the pH of water by researchers in the field and scientists in the lab. Potentiometric devices, such as a portable pH meter for water or lab-based devices, use the electrical potential differences of pH-sensitive hydrogen, metal, or glass electrodes placed in control and test samples to detect the pH of liquids. This picture shows a typical portable pH meter.

For regular consumers, optical or visual methods of estimating pH are more appropriate and easier to use. These methods use strips (litmus strips) that contain pH-sensitive organic pigments, called indicators. When a sample of water is applied to these test strips, they will change color. The pH value then can be roughly estimated by comparing the color of the test strip against a provided color scale. This picture shows a typical pH test strip and color chart.

What Are the Safe pH Ranges of Drinking Water?
The safe pH ranges of drinking water have not been established in binding regulations because the pH of all known, properly regulated tap and bottled drinking water has proven safe. According to the World Health Organization (WHO) Guidelines for Drinking-water Quality, it is not considered necessary to propose health-based guideline ranges for drinking water.
A range of 6.5-9.5 pH of water is often arbitrarily given as the best pH for drinking water. This range is thought to have minimal effects on drinking water quality or safety. The Canadian Drinking Water Guidelines and the United States National Secondary Drinking Water Regulations (NSDWRs) both recommend that drinking water should be discharged between a pH of 6.5 and 8.5. This is based on WHO recommendations not for health specifically but for ensuring chlorine disinfection functions properly and metallic corrosiveness is minimized.
pH alone is not the primary determinant of drinking water safety. The acids and bases which determine the pH of water are extremely weak and dilute, posing no threat to human health. For example, gastric acid (HCl) in the stomach is a strong acid that degrades most biological matter we consume and has a pH of 1.5-3.5. Lemon juice and vinegar have similar pH values at 2.4 and 2.8, but they are weak acids and do not cause any harm when consumed. The sample principle applies to acidic and alkaline drinking waters which by themselves pose no harm and are safe.
What Are the Harms of High pH Drinking Water?
There is no scientific evidence that there is any harm associated with high pH drinking water. High pH drinking water is known as alkaline or basic. These waters have a pH greater than 7, with most alkaline waters having an advertised pH between 8-10.
A 2016 Health Canada technical report on drinking water found limited evidence that exposure to extremely alkaline water (pH greater than11) may cause skin or eye irritation. This is beyond the pH range of any of the 190 bottled waters for which the Fine Water Society has profiles, the highest of which is the 10.01 pH of FOZ Natural Alkaline Water from Brazil.
The WHO reports that high pH negatively impacts the disinfection process of drinking water. Effective disinfection of water with chlorine requires a pH of less than 8. This could potentially lead to the unsatisfactory treatment of drinking water, which may harm consumer health.
Lastly, it is important to point out that despite numerous marketing claims, there is insufficient scientific evidence that high pH or alkaline water is beneficial to one’s health or is used to remedy particular health conditions.
What Are the Harms of Low pH Drinking Water?
The harms of low pH drinking water refers to the possible health harms to the human body caused by low pH (generally under a pH of 4) of drinking water.
There is no scientific evidence that there is any harm associated with low pH drinking water. Low pH drinking water is known as acidic. These waters have a pH less than 7, with most acidic waters having a pH between 6-6.9.
Although not directly harmful to consumer health, acidic water may cause indirect dangers to individuals depending on its environment. Low pH affects the degree of corrosion of heavy metals into water and could thus contain higher concentrations of toxic metals such as lead which lead to heavy metal toxicity if consumed in large quantities or for long periods of time. However, tap and bottled water is rigorously tested in most countries to ensure such metal levels are not present, meaning it is rarely an issue.
When the pH of water drops below 4, it leads to eye and skin irritation, but there is no known bottled or tap water available at these levels.
How to Lower the pH of Drinking Water
The simplest way to reduce the pH of drinking water is to add a few drops of lemon juice. The citric acid in lemon juice will naturally reduce the pH.
Ion exchange filters, whether attached at the faucet or under the sink, reduce the pH of water by removing alkaline minerals such as calcium, manganese, and fluoride.
If more drastic measures are needed, a home acid injection system is installed.
What Are Common Drinking Water pH Levels?
These are common drinking water pH levels for different types of water.
- Tap water. Highly variable, average pH 7.5
- Distilled water. pH 5.8 - 7
- Bottled water. Highly variable based on source, pH 5 - 10
- Ocean water. pH 8 - 8.1
- Sparkling water. pH 4.5 - 6
- Iceberg water. pH 6 - 8
- Mineral water. pH 5.5 - 8.5
- Spring water. pH 6.5 - 8.5
- Artesian water. Highly variable based on ground geology, pH 6.5 - 8.5
- Alkaline water. pH 8-10
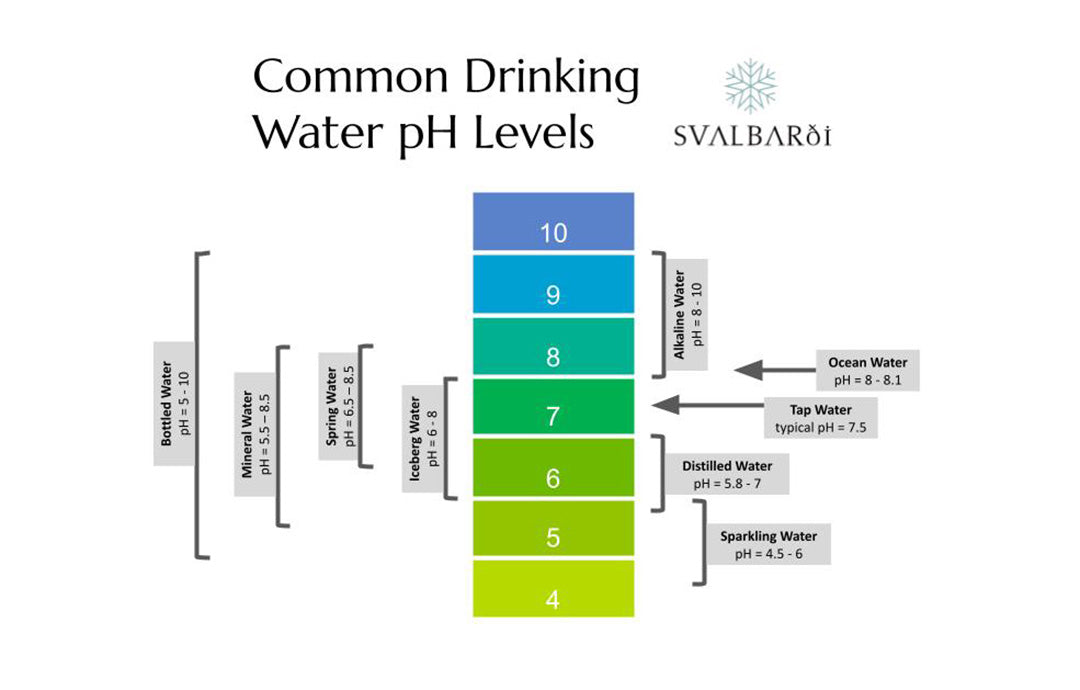
With the exception of ocean water, all of these water types are used for daily drinking, though sparkling, mineral, and especially iceberg water tend to be used more in dining settings. Even ocean water is drunk if desalinated as it is in many countries and a few bottled water brands. The pH range is wide and not of any scientific meaning but is sometimes utilized for marketing purposes.
1. Tap Water
Tap water refers to water that is derived from a public water system and is distributed through pipes or other constructed conveyances.
The pH of tap water is highly variable as it depends on its source, processing, and delivery system. As such pH tap water throughout the world will be different. But if the pH level goes outside of certain ranges, is tap water safe to drink?
The US Environmental Protection Agency and the World Health Organization recommend that the pH range of tap water meant for drinking should be maintained between 6.5-9.5. This range is somewhat arbitrary, with the agencies acknowledging there is no specific health basis behind it. There are no known cases where the pH level alone caused tap water to be unsafe.
However, the recommended range of 6.5 to 9.5 pH for tap water does reflect the reality that most systems try to keep their pH above 7 to minimize corrosion effects in their treatment plants and pipe distribution networks.
For comparison between tap waters, these are some pH values from past winners of the municipal water category of the annual Berkeley Springs International Water Tasting competition.
|
Municipality |
Award |
Average pH |
Range of Detections |
|
Metropolitan Water District of Southern California, USA |
2021 Gold |
8.2 |
8.1 - 8.5 |
|
Santa Ana, California, USA |
2021 Silver |
7.9 |
7.6 - 8.1 |
|
Clearbrook, BC, ada |
2019 Gold |
7.4 |
7.0 - 7.8 |
|
Hamilton, Victoria, Australia |
2019 Silver |
7 |
6.4 - 7.6 |
|
Eldorado Springs, Colorado, USA |
2016 Gold |
7.72 |
7.69 - 7.72 |
2. Distilled Water
Distilled water is created by boiling any water source into vapor and then collecting the condensation into a separate container.
The pH of distilled water ranges from 5.8 to 7. For example, Coca-Cola-owned Smartwater is a distilled bottled water brand claiming a “balanced” pH of 7.0, but their competitor Waikea states Smartwater has a pH of 6.5.
Pure distilled water should have a pH of 7 having theoretically had all components other than pure H2O removed. In reality, depending on the water source, some trace elements that boil off at temperatures up to or below that which is being used for the water may remain. As soon as the water is removed from a sealed distillation system, it will begin to absorb atmospheric gasses including CO2, and start to become acidic.
Distillation removes all oxygen from water as well as minerals, producing pure but flat-tasting demineralized water. Although safe to drink, the beneficial trace minerals that water normally contains are absent from distilled water and it is essentially hypotonic. This could potentially worsen any mineral deficiency an individual may have and the hypotonicity may promote excessive urination and loss of important electrolytes.
3. Bottled Water
As defined by the US Food and Drug Administration (FDA), bottled water refers to water intended for human consumption and sealed in bottles or other containers intended for sale. With such a broad definition, the pH of bottled water is highly variable depending on its source and brand.
These are the main categories of bottled water and some typical pH levels and brand examples. Typical ranges for all-natural source waters are based on the Fine Water Society’s collection of brand profiles.
|
Category |
Source Type |
Typical pH Range |
Brand Example |
|
Distilled water |
Processed |
5.8 - 7 |
Smartwater (pH 6.5 - 7) |
|
Purified water |
Processed |
5 - 8 |
Aquafina (pH 5.5 - 7) |
|
Mineral water |
Natural |
7 - 8.5 |
Hildon (pH 7.2) |
|
Spring water |
Natural |
6.5 - 8.5 |
Poland Spring (pH 6.1 - 7.2) |
|
Artesian water |
Natural |
6.5 - 8 |
Fiji (pH 7.7) |
|
Iceberg water |
Natural |
6 - 8 |
Svalbarði Polar Iceberg Water (pH 6) |
|
Alkaline water |
Processed |
8 - 10 |
Essentia (pH 9.5) |
|
Rainwater |
Natural |
6.5 - 7.5 |
Cloud Juice (pH 7.3) |
4. Ocean Water
According to the US Environmental Protection Agency, the current average pH of ocean water is 8.1. It ranges from 7.5 to 8.4 and before the Industrial Revolution the average was 8.2. Due to the logarithmic nature of the pH scale, this means that the ocean is now 25% more acidic than before industrialization.
Ocean water has a very high salt content. Humans are not meant to consume ocean water, as it introduces very high amounts of sodium chloride which disrupts the very careful ionic balance required for normal functioning of cells. To maintain this balance, the kidneys have to eliminate the excess salt. The only way this is accomplished is by inducing saline diuresis, through which kidneys excrete large amounts of urine to eliminate the excess salt. This state leads to rapid dehydration and even death.
Desalinated water is one form of ocean water that is consumed. Countries with scarce water supplies such as Saudi Arabia, Singapore, and Spain use desalinated sea and ocean water. This process removes sodium chloride and other mineral components from ocean water and makes it safely drinkable. The pH of desalinated water is initially around 5.5 but is treated to raise it to around 8. such as Kona Deep in Hawaii use the process of reverse osmosis to take salt out of Hawaiian ocean water and subsequently bottle and market it with a pH of 6.7.
5. Sparkling Water
Sparkling water refers to water that either naturally or artificially contains a high amount of carbon dioxide, making it “bubbly”. They typically have a pH between 4.5 and 6 due to the extra acidity caused when carbon dioxide converts to carbonic acid in water. They tend to have higher mineral concentrations than still waters because they either emerge from mineral-rich volcanic regions or have bicarbonate added to reduce the acidity of artificial carbonation.
The pH values for some sparkling water includes the following well-known brands.
- San Pellegrino. pH 5.6
- Vichy Catalan. pH 6.82
- Perrier. pH 5.5
- Badoit. pH 6
- Gerolsteiner. pH 5.9
- Voss Sparkling. pH 4.8

Even though sparkling water brands are slightly acidic, they are nonetheless safe for consumption. Keep in mind that soft drinks like Coke have a pH of 2.4, which is much lower than that of any sparkling water brand.
6. Iceberg Water
Iceberg water comes from small iceberg pieces which naturally calve off glaciers into the ocean. Shortly before they would otherwise melt into the sea, they are collected, melted, bottled, and generally sold as luxury water.
Because icebergs are made from fresh snow which fell hundreds or thousands of years ago before being quickly preserved as compacted ice, iceberg waters in their original state are free of modern pollutants and have the pH of clean precipitation. According to the US EPA clean rainwater averages a pH of 5.6. It is slightly acidic because when it first fell as snow it absorbed natural gases from the atmosphere. These give it a fresh airy taste but do drop the pH somewhat.
Some iceberg water brands choose to raise the pH using processes such as activated carbon filtration. These alter the taste and chemistry from the iceberg water’s natural state.
There are very few brands of iceberg water on the market and there are a few that are no longer in operation. These are the most well-known iceberg water brands, past and present.
- Svalbarði Polar Iceberg Water. pH 6
- Iluliaq (defunct). pH 7.8
- Berg. pH 7.8
- Glace (defunct). pH 7.8
- IceBerg Water. pH 7.2

7. Mineral Water
Mineral waters come from underground sources that are tapped at natural or borehole exit points and have stable concentrations of minerals and trace elements. They are highly regulated to ensure they are sold in the natural state from which they emerge from the ground with all their healthy minerals unchanged. The EU Drinking Water Directive allows very few purification methods, insisting that the water be pure at source and when bottled, with constant testing requirements to ensure safety. Mineral waters are still or sparkling, with carbon dioxide to make sparkling versions of naturally still waters the only allowable additive.
Because mineral waters include both sparkling and still waters as well as a wide range of mineral compositions, their pH levels vary widely. Out of 69 medium to high TDS (total dissolved solids, the amount of minerals dissolved in water, measured in milligrams per liter) mineral waters covered by Fine Water Society brand profiles, 90% have a pH between 5.5 and 8.5, and 61% a pH between 6 and 8. These are the pH values of some particularly interesting mineral water brands.
- Three Bays (Australia). pH 8.3
- Evian (France). pH 7.2
- ROI (Slovenia). pH 6.8
- Pedras (Portugal). pH 6.1
- FOZ Natural Alkaline Water (Brazil). pH 10
- Staatl. Fachingen (Germany). pH 5.8
- Aqua Carpatica (Romani). pH 6.6
8. Spring Water
Spring water refers to water that comes from an underground formation that naturally flows to the surface and is collected or is alternatively tapped. Unlike mineral water, it does not necessarily have to have a stable mineral content. This means that mineral waters are usually spring waters as well, but spring waters are not necessarily mineral waters.
So long as they are properly tested to meet quality parameters, all spring waters on the market are safe to drink. Some municipal tap water systems utilize springs as a source.
The diverse array of carbonation levels and natural sources of spring waters means that they have a wide range of pH values. Out of 116 spring waters covered by Fine Water Society brand profiles, 72% have a pH between 6.5 and 8.5. While strict regulation and premium marketing mean “mineral water” brands are more premium, looser definitions for spring water mean both mass-market and premium brands fill the space.
Mass-market bottled water brands often use multiple sources, meaning the pH range is very wide. This table shows some typical examples.
|
Brand |
Type |
Country |
pH |
|
Poland Spring |
Mass-market |
USA |
6.1 - 7.2 |
|
Deer Park |
Mass-market |
USA |
6.6 - 8.2 |
|
Kirkland Signature Spring Water |
Mass-Market |
UK |
7.5 |
|
Icelandic Glacial |
Premium / Mass-market |
Iceland |
8.7 |
|
Lahuenco |
Premium |
Chile |
9.5 |
|
Água Castello |
Premium / Mass-market |
Portugal |
5.35 |
9. Artesian Water
Artesian water is collected from wells that tap aquifers that contain water under pressure and when tapped push the trapped water towards the surface. If they have a stable mineral composition and water quality parameters, they qualify as mineral waters.
The diverse array of carbonation levels and natural sources of artesian waters means that they have a wide range of pH values. Out of 40 artesian waters covered by Fine Water Society brand profiles, 75% have a pH between 6.5 and 8.5. These are the pH values of artesian water brands around the world.
- Fiji. pH 7.7
- Primal (South Africa). pH 7.7
- Bodh (Bhutan). pH 7.2
- Magnificat (Portugal). pH 5.1
- Babulong (Taiwan). pH 8.75
- 420 (New Zealand). pH 7.8
- Manantiales Sureños (Paraguay). pH 9.48
10. Alkaline Water
Alkaline water refers to any water from any source that has a pH above 7, though in bottled water markets it is usually used for brands with a pH of 8 to 10. It has become a popular term implying health benefits (which are not supported scientifically), so some brands that have a pH in the 7’s use it as well for marketing purposes.
The pH values of some water brands that are marketed as “alkaline” include these.
- Essential: pH 9.5
- Flow: pH 8.1
- Core: pH 7.4
Alkaline water is neither dangerous nor does it have scientifically established positive health benefits, so it is consumed in the same way as any other type of drinking water.

Charlotte, just get a pH reagent, it’s a liquid. Just pour 2-3 drops on 30ml of the bottled water you wish to test. I have tried it on different bottled water. It’s amazing.
I’m not familiar with that brand however if I was looking for the pH of something and was unable to find it with research online I read buy pH strips and test it myself. Even though labels and the internet may say one thing testing it yourself is easy and I think settles better with the mind wondering if what they say is actually what it is rather you know because you tested it! They’re cheap and easy to buy online if you cannot find them in your local market.
Simply dip the paper strip in whatever you’re testing wait a minute and after it turns color compare it to the chart on the side of test strip container and there it is you have the pH of whatever you’re in question of.
I’m trying to find the ph level for the bottled water – Fruit 2o – but I can’t. Can you advise where I can find it?
Leave a comment